Strategic consultancy to support expanding indications for vaccines
Developing and managing a comprehensive communications plan to provide strategic recommendations to support expanding indications across two vaccine products
The challenge
Our client had an extensive program of HEOR medical communications to support the expanding indications for two established vaccine products which required ongoing review and management. To align with ongoing evidence generation, our client wanted to ensure timely publication of medical communications to support their HEOR communication strategy.
The solution
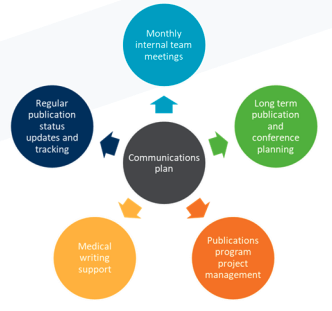
We developed a comprehensive HEOR communications plan, including aligning publications to key clinical milestones, coordinating client internal processes and providing medical writing support across the portfolio. To do this, we:
> Consolidated publication plans and regular updates across the program into a single manageable database.
> Tracked planned communications against upcoming milestones and target conference dates to provide strategic recommendations.
> Provided ad-hoc medical communications support.
Key results
We provided a clear, comprehensive publication tracking database with regular updates collated across the internal client team and with each publication clearly linked to the overall product strategy so there was clarity on which aspect of the strategy each HEOR publication was supporting. Bespoke reports and visual decks allowed communication of ongoing publication successes and future plans. Expert medical writing support was provided across publications.
Value to the client
The internal client team was able to quickly identify key publication information and better understand and align their publication planning to support product expansion into new indications, and also to clearly communicate how the HEOR publications were aligned to and contributing to the overall product strategy.