Delphi panel to elicit expert consensus relating to the assessment of European Immunization programmes in paediatrics
Insights obtained from scientific experts within paediatric infectious disease to validate the scoring system of a tool developed to assess national immunization programmes (NIPs) in Europe
The challenge
Our client had developed an internal tool designed to provide an up-to-date picture of current European paediatric NIPs and to assess the comprehensiveness of paediatric vaccination calendars across Europe. They sought to further develop and validate the tool before communicating the findings externally. The overarching aim was to highlight what gold standard practice looks like and to help support and further the evolution of European paediatric NIPs.
The solution
We conducted a Delphi panel to validate the selection and weighting of elements included in the current immunization program comprehensiveness scoring tool. We worked in close collaboration with our client and a steering committee, comprised of three Key Opinion Leaders (KOLs) within the paediatric infectious disease landscape.
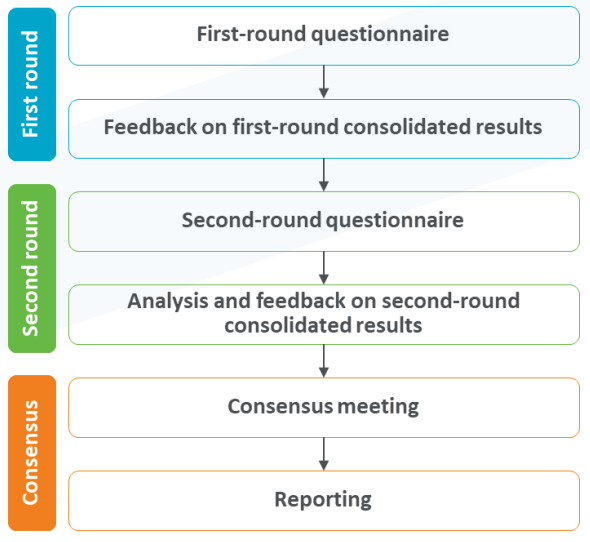
Key results
Two online surveys and one consensus meeting with KOLs across Europe were completed. Consensus was achieved on the approach adopted for the assessment and validation of the scoring system implemented within the tool. Key consensus recommendations were obtained from experts within the paediatric infectious disease landscape in terms of potential ways to improve the tool and the scoring system applied. A detailed study report and peer-reviewed manuscript was developed summarizing the key outputs of the Delphi panel, which enabled our client to communicate the utility of the tool and what gold standard practice looks like to help support the evolution of NIPs across Europe.
Value to the client
The completion of the Delphi panel ensured the scoring system implemented within the paediatric vaccination programme comprehensiveness assessment tool was validated and appropriate for use based on the opinions of KOLs within the paediatric infectious disease space. The development of a peer-reviewed manuscript also ensured that our client was able to disseminate the findings relating to the performance of NIPs across Europe to promote evolution of NIPs across key markets.

