A network meta-analysis of randomised controlled trials in dementia with Lewy bodies
Evaluating the efficacy and safety of current therapies in an unapproved indication
The challenge
Many treatments for Alzheimer’s disease are also used to treat dementia with Lewy bodies (DLB), however only the client’s was approved for this use (and in only one market). Our client wished to evaluate the outcomes of the available treatment options based on currently used trial endpoints in order to improve their understanding of differences between existing and potential future treatment options, including their own pipeline compounds.
The solution
In order to produce comparative evidence between available treatment options, we developed a network meta-analysis (NMA) reviewing the clinical efficacy and safety of cognitive-behavioural treatments that were commonly used in patients with DLB. The NMA was conducted based on the findings of a previously published systematic literature review (SLR) reporting on pharmacological interventions. A feasibility assessment was carried out to confirm that indirect comparisons between DLB pharmacological interventions were viable for the proposed outcome measures, and that there were no new data likely to be published within two to three years that may impact the NMA.
Key results
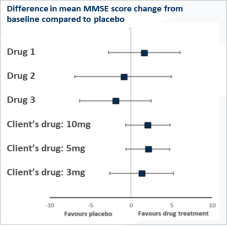
While very few results produced were statistically significant, the trends suggested our client’s current treatment had a more favourable overall benefit/risk profile for patients with DLB than the other available treatment options.
Value to the client
Although the conclusions were supported by consistent trends across all included outcome measures, the lack of significant results suggested that additional clinical trials would be required to strengthen the analysis. In addition, the variability across patient groups within each trial led to significant heterogeneity between the different studies included in the analysis. Read our published abstract: https://pubmed.ncbi.nlm.nih.gov/32495063/
